Health Department Refrigeration Monitoring
Both public and private organizations that are required to monitor the safety of food and drugs consider proper refrigeration temperature management of vital importance.
Temperature monitoring of drugs, biologics or food are not only vital to assure safety but are also required by a variety of governmental authorities including local, state or federal health departments. Plus, your company may also have policies and procedures in place to protect your refrigerated inventory. The following are general considerations to assure you’re maintaining the proper storage procedures; contact your immediate supervisor or regulating agency for more information.
To assist you in understanding some of the various oversights, here is a quick summary. Please call us if you have any further questions.
Units should be checked at least twice per day. We would always recommend a check is carried out as soon as possible at the beginning of your working day or better yet — invest in a remote monitoring system to automate temperature record keeping and alert you via smart phone when your temperature is out of your pre-determined limits, or your unit is predicted to have an issue (excursion) in the future.
HEALTHCARE
AHJ: The local Authority Having Jurisdiction (AHJ) is an organization, health department, or individual responsible for enforcing the requirements of a code or standard, or for approving equipment, materials, an installation, or a procedure.
JCAHO: A process must be in place to ensure appropriate temperatures are being maintained for the medications stored. Organization should also have a defined process for the disposal of medication from a refrigerator or freezer which has deviated from the specified temperature range.
Consult with state and local authorities having jurisdiction including local, state or federal health departments to address regulations and requirements specific to your geographic location.
The Joint Commissions standards require that organizations store food and nutrition products, including those brought in by patients or their families, using proper sanitation, temperature, light, moisture, ventilation, and security as per PC.02.02.03.
The Joint Commission does not specifically require temperature logs for refrigerators and freezers used for food storage. Standard CTS.04.03.33 requires that food and nutrition products are stored under proper conditions of sanitation, temperature, light, moisture, ventilation, and security. These variables must be monitored to assure proper storage.
CDC: Proper vaccine storage and handling practices play a very important role in protecting individuals and communities from vaccine-preventable diseases. Vaccine quality is the shared responsibility of everyone, from the time vaccine is manufactured until it is administered.
The Vaccine Storage and Handling Toolkit has been updated with a COVID-19 Vaccine Addendum with information on Storage and Handling best practices for COVID-19 vaccines. All vaccination providers participating in the COVID-19 Vaccination Program must store and handle COVID-19 vaccines under proper conditions to maintain the cold chain as outlined in the toolkit and addendum. This addendum will be updated with specific storage and handling information for each COVID-19 product.
FOOD SAFETY
FDA: About 48 million people in the U.S. (1 in 6) get sick, 128,000 are hospitalized, and 3,000 die each year from foodborne diseases, according to recent data from the Centers for Disease Control and Prevention. This is a significant public health burden that is largely preventable.
Most fresh foods must be stored in the refrigerator to delay their deterioration and decomposition. The most basic rule must be always followed: store raw products below, never above, your cooked or ready-to-eat products. Keep your appliances at the proper temperatures. Keep the refrigerator temperature at or below 40° F (4° C). The freezer temperature should be 0° F (-18° C). Check temperatures periodically.
The FDA Food Safety Modernization Act (FSMA) is transforming the nation’s food safety system by shifting the focus from responding to foodborne illness to preventing it. Congress enacted FSMA in response to dramatic changes in the global food system and in our understanding of foodborne illness and its consequences, including the realization that preventable foodborne illness is both a significant public health problem and a threat to the economic well-being of the food system.
According to U.S. Food and Drug Administration (FDA) CFR – Code of Federal Regulations Title 21: All operations in the receiving, inspecting, transporting, segregating, preparing, manufacturing, packaging, and storing of food shall be conducted in accordance with adequate sanitation principles.
Food shall be stored under conditions that will protect against contamination and minimize deterioration: maintain refrigerated foods at 45 deg.F (7.2 deg.C) or below as appropriate for the particular food involved and retain frozen foods in a frozen state.
All food manufacturing, including packaging and storage, shall be conducted under such conditions and controls as are necessary to minimize the potential for the growth of microorganisms. One way to comply with this requirement is careful monitoring of physical factors such as time, temperature, humidity, etc. Monitoring of refrigeration is required to ensure that mechanical breakdowns, time delays, temperature fluctuations, and other factors do not contribute to the decomposition or contamination of food.
Inventory should be monitored to protect contamination requires using adequate time and temperature controls.
Hazard Analysis and Critical Control Points (HACCP) is a management system that deals with and creates food safety guidelines that must be followed wherever food is displayed, stored, prepared, cooked and/or moved. It recognizes that the most important factors for food safety and quality are pH and temperature. Foods that can allow bacteria to proliferate when stored improperly are deemed high-risk and include the following: chicken, meat, seafood, dairy products, eggs, etc. Salad vegetables and foods prepared with any of the ingredients are deemed high-risk as well. Food with temperatures that range from 5 degree Celsius to 60 degrees Celsius allows bacteria the chance to grow at excessive rates.
The OneEvent® System® is an efficient way to maintain the safety of food products and help assure consumer health. Plus, with automated record keeping and reporting, time and money are saved by eliminating manual efforts.
Free, No-Obligation Quote
Contact us today for a free, no-obligation customized quote based on your specific needs.

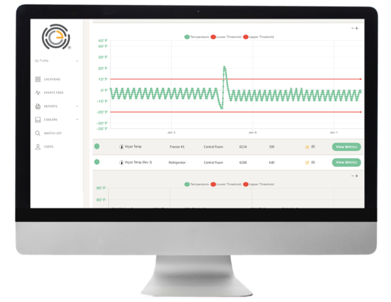
OneEvent delivers patented technology that protects refrigerated inventory through predictive-analytics and is shifting the industry paradigm from reaction to prevention.
- The OneEvent refrigeration monitoring system is wireless and cellular based with WiFi access available
- Wireless sensors provide reliable input 24/7/365
- Secure cellular gateway with battery back-up to assure reliability
- The OneEvent patented Thermo Heartbeat®displays real-time health report of all the refrigeration units and CAN ACTUALLY PREDICT REFRIGERATION BREAKDOWNS WEEKS IN ADVANCE!
- Receive real-time smartphone alerts when temperature or door open times exceed parameters
- OneEvent’s patented analytics and IoT technology turns your data into actionable information
- Comprehensive dashboard access via any computer or tablet includes average temperature live view, customized reporting, watch list and individual unit make, model and age
- Schedule alerts for NIST certification and calibration
- Historical data storage and exports to replace paper logs
- Meets all CDC requirements with optional VFC compliant system available
- Detailed excursion reporting
To comply with VFC/CDC and COVID Vaccine requirements, OneEvent includes a vaccine storage digital datalogger (DDL) in conjunction with the standard OneEvent refrigeration temperature monitoring system.
- The OneEvent DDL is rugged, durable, splash proof and powered for up to 1-year with field replaceable batteries
- Readings are stored in cloud for easy access
- Range to -40°C/-40°F. With required buffered temperature probes with food-safe glycol for stable readings
- Includes a 2-year NIST-Traceable calibration certificate

CONTACT US
Find out more about how OneEvent® System can help you.
Call 844.485.0880 | Email
or Click the Button Below
