Biomedical and Laboratory Wireless Refrigeration Monitoring
Utilizing cold storage best practices will empower biomedical and laboratory facilities to ensure refrigerated inventory is compliant while providing advance notification prior to a system breakdown.
The Centers for Disease Control and Prevention (CDC) Guidelines mandate cold chain management. Proper storage of medical inventory is critical because any loss of potency may negatively affect safety and efficacy and compromise biomed and laboratory research results.
Not maintaining proper temperatures and the resulting excursions are the most common cause of the degradation of biomed and lab inventory. For example, a vaccine can survive higher temperatures for a minimal period of time and still be deemed safe, but its lifespan may be shortened. Refrigerated vaccines are more susceptible to damage when temperatures dip below freezing. Refrigerators need to be carefully monitored to ensure that all biomed and lab inventory is kept within appropriate temperature ranges.
The Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations (JCAHO)) does not specifically require temperature logs for freezers and refrigerators, but it does stipulate that inventory be stored according to the manufacturer’s recommendations and that facilities maintain and monitor equipment performance.
Facilities must track temperatures on a regular basis and a process must be implemented for disposing of medication that no longer meets the recommended temperature ranges.
There are different methods with which to measure temperature inside a refrigerator or freezer in biomed and lab environments. Thermometers are the most basic. OneEvent’s sensors measure temperature and connect wirelessly to a OneEvent Gateway that continually displays and records the temperature readings.
According to the Centers for Disease and Control and Prevention (CDC) Vaccine Storage and Handling Toolkit, thermometers used to monitor vaccine temperature should be:
- Equipped with a remote probe and suitable for the temperature range of the application
- Submerged in glycol or glass beads
- Calibrated with a Certificate of Traceability
CDC recommendations include the use of data-loggers (DDL) in certain biomed and lab applications including:
- Digital display on the outside of the storage unit for reading temperatures without opening the door
- Detachable
- Probe in a bottle filled with a thermal buffer, like glycol, which more closely reflects vaccine temperatures; vaccine temperatures have been found to be more thermo-stable than air temperature, which fluctuates with defrost cycles and opening and closing of the unit door
- Alert for out-of-range temperatures
- Accuracy to within ±1 °F (±5 °C)
- Low-battery indicator
- Continuous monitoring and recording capabilities to track and record temperatures over time
- Display of current, minimum and maximum temperatures, which indicate the coldest and warmest temperatures recorded since the device was reset.
The OneEvent® application can also support other needs for life sciences and clinical research environments including room temperature, humidity, motion, door open/close, carbon monoxide and flood/water detection.
Free, No-Obligation Quote
Contact us today for a free, no-obligation customized quote based on your specific needs.

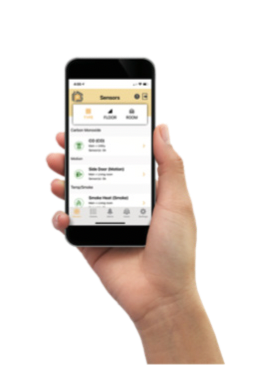
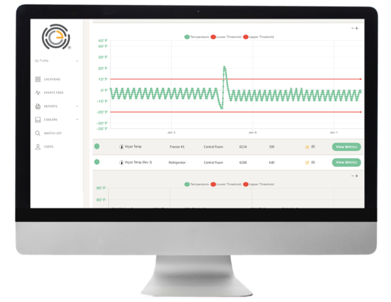
OneEvent delivers patented technology that protects refrigerated inventory through predictive-analytics and is shifting the industry paradigm from reaction to prevention.
- The OneEvent refrigeration monitoring system is wireless and cellular based with WiFi access available
- Wireless sensors provide reliable input 24/7/365
- Secure cellular gateway with battery back-up to assure reliability
- The OneEvent patented Thermo Heartbeat®displays real-time health report of all the refrigeration units and CAN ACTUALLY PREDICT REFRIGERATION BREAKDOWNS WEEKS IN ADVANCE!
- Receive real-time smartphone alerts when temperature or door open times exceed parameters
- OneEvent’s patented analytics and IoT technology turns your data into actionable information
- Comprehensive dashboard access via any computer or tablet includes average temperature live view, customized reporting, watch list and individual unit make, model and age
- Schedule alerts for NIST certification and calibration
- Historical data storage and exports to replace paper logs
- Meets all CDC requirements with optional VFC compliant system available
- Detailed excursion reporting
To comply with VFC/CDC and COVID Vaccine requirements, OneEvent includes a vaccine storage digital datalogger (DDL) in conjunction with the standard OneEvent refrigeration temperature monitoring system.
- The OneEvent DDL is rugged, durable, splash proof and powered for up to 1-year with field replaceable batteries
- Readings are stored in cloud for easy access
- Range to -40°C/-40°F. With required buffered temperature probes with food-safe glycol for stable readings
- Includes a 2-year NIST-Traceable calibration certificate

CONTACT US
Find out more about how OneEvent® System can help you.
Call 844.485.0880 | Email
or Click the Button Below
